The DHIS2 Annual Conference takes place from 15-18 June 2026! Learn more
PAHO pilots DHIS2 to strengthen vaccine safety in the Americas
In the context of the COVID-19 pandemic and vaccine introduction efforts, the Pan American Health Organization has focused on strengthening pharmacovigilance following immunization.
According to the Pan American Health Organization’s (PAHO) Manual for the surveillance of Events Supposedly Attributable to Vaccination or Immunization in the Region of the Americas, an ESAVI (Event Supposedly Attributable to Vaccination and Immunization) is “any untoward medical occurrence which follows immunization and which does not necessarily have a causal relationship with the usage of the vaccine. The adverse event may be any unfavorable or unintended sign, abnormal laboratory finding, symptom, or disease.” It is also known as AEFI (adverse event following immunization) or MAPI in other regions. If an ESAVI is not rapidly and effectively dealt with, it could delay the identification of risks related to vaccines or can undermine confidence in a vaccine and ultimately have dramatic consequences for immunization coverage and disease incidence, when coincidental events are wrongly attributed to the vaccine.
The PAHO ESAVI project aims at developing a sensitive, timely, standard, reliable and integrated ESAVI surveillance system for the pan-American region, with the involvement of all stakeholders including National Immunization Programs, National Regulatory Authorities, Epidemiological Surveillance Units and other units at the Ministries of Health.
“This initiative will help maintain confidence in vaccination and acceptance of immunization in the Americas”
The need for a versatile digital platform for monitoring ESAVI
At the beginning of 2020, 62% of the countries in the PAHO region that work in surveillance used paper and spreadsheet-based systems. Only 17% of existing solutions for ESAVI reporting are centralized web systems. The remaining 21% have fragmented and isolated systems. Public skepticism of the new COVID vaccines and a need for transparency and swift information flows have increased country demand for a flexible system that can interoperate and use vaccine safety standards. Countries are also expected to report to regional and global vaccine safety databases. At the same time, the goal of strengthening countries’ information systems should help increase the quality and usefulness of data as well as reduce the administrative burden.
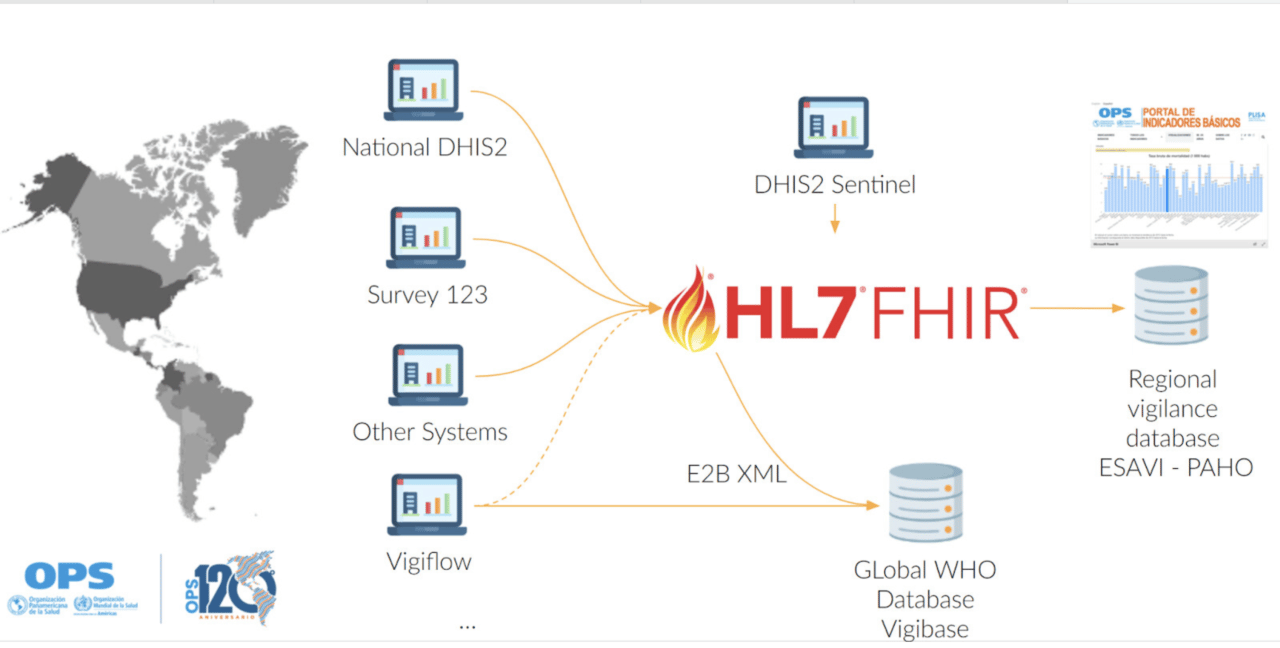
PAHO has adapted and expanded the DHIS2 AEFI Tracker Package to fulfill regional guidelines and is currently running its own instance for reporting adverse events in sentinel sites. This new package adds a new stage to the investigation process and includes clinical terminology dictionaries as well as pharmaceutical standards.
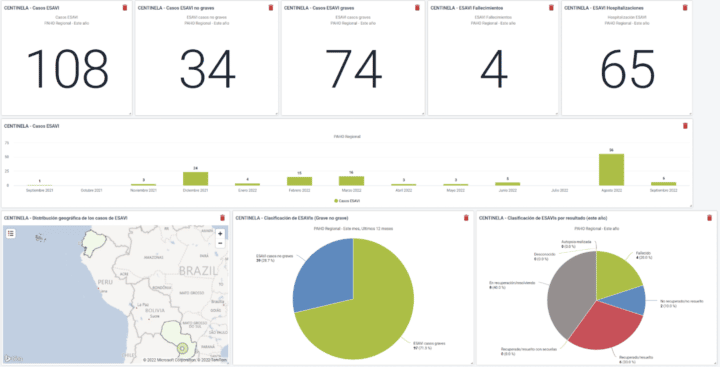
Using the system, countries can report directly into this instance from sentinel hospitals and contribute to the regional database. This also serves as a testing and piloting ground for countries considering their own national implementation for broader ESAVI surveillance. The program has been standardized and is currently in the process of being packaged and is offered to countries that want their own ESAVI implementation. Currently, Brazil and Paraguay are already entering data in the PAHO regional instance, and several other countries are considering joining the regional implementation or starting their own.
Supporting multilateral pharmacovigilance efforts by interconnecting regional databases using DHIS2
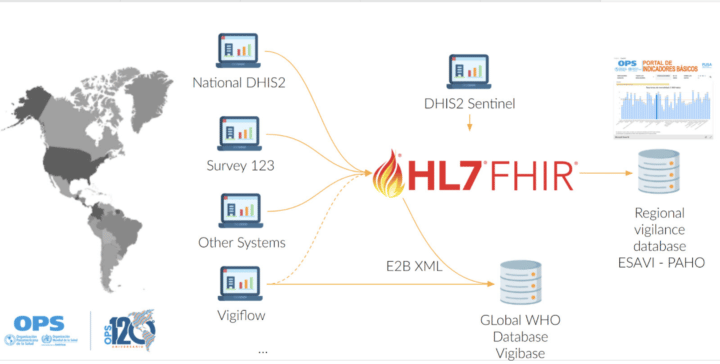
The DHIS2 ESAVI program has been adapted to include WHODrug and MedDRA as coding standards, as it would ease the data exchange and analytics process. Nevertheless, countries can include the terminology standard that better suits their needs, including ICD-11 or SNOMED.
Moreover, PAHO aims at maintaining a FHIR server and an ESAVI FHIR implementation guide to support interoperability between the regional pharmacovigilance database, the global pharmacovigilance database VigiBase, DHIS2 and other country systems.
Additional information
If you have questions or comments about this project, you can discuss them with the team on the DHIS2 Community of Practice.
If you want to know more about this check out the presentation “Digital Transformation of Vaccine Safety in the Americas” from the DHIS2 annual conference 2022.
If you are interested in other PAHO projects with DHIS2, vigilance, or DHIS2 in the Americas you can also watch the recording of the DHIS2 Annual Conference presentation about Surveillance of Vaccine-preventable diseases.